Description
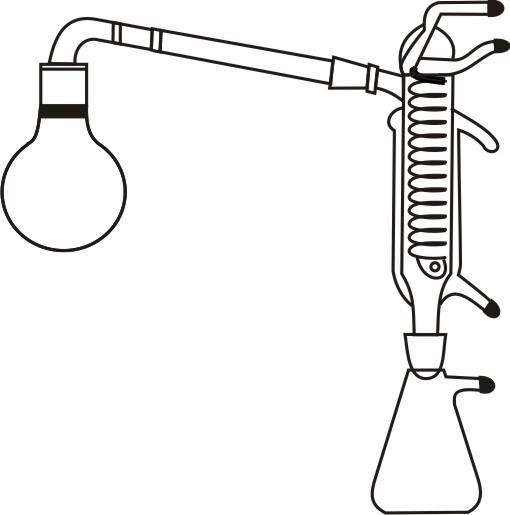
The MEDILAB Solvent Recovery Assembly is a complete laboratory setup designed to recover, purify, and recycle solvents through controlled distillation. It consists of a 500 mL round-bottom flask, a sloping recovery bend, a 200 mm air condenser, an ether condenser, and a 250 mL Buchner flask. This assembly ensures efficient vapour condensation and solvent collection, making it essential for laboratories working with costly, sensitive, or volatile solvents. It enhances operational efficiency while reducing solvent waste and overall laboratory cost.
Key Features
- Comprehensive recovery system engineered for accurate solvent recycling and purification.
- Made from Borosilicate Glass 3.3, offering superior thermal and chemical resistance.
- A dual condenser setup (air condenser + ether condenser) ensures maximum cooling efficiency for low-boiling solvents.
- Sloping recovery bend promotes smooth vapour flow and prevents reflux blockages.
- High-clarity glass provides clear visibility of vapour movement, condensation, and solvent collection.
- Standard ground glass joints ensure airtight sealing and compatibility with most laboratory distillation equipment.
- Thermal shock resistant—ideal for repeated heating and cooling cycles.
- Durable construction with uniform wall thickness for long-term laboratory use.
- Ideal for solvent-saving workflows, reducing chemical consumption and environmental impact.
Material Specifications
- Material: Premium Borosilicate Glass 3.3
- Thermal Properties: Low thermal expansion coefficient (3.3 × 10⁻⁶ K⁻¹). Withstands high temperatures and rapid cooling. Suitable for continuous distillation operations
- Chemical Resistance: Excellent compatibility with organic solvents, acids, and laboratory reagents. Non-reactive, ensuring pure and contamination-free recovery
- Mechanical Properties: High strength and stress-relieved via controlled annealing. Resistant to mechanical wear in everyday lab handling
- Standards & Compliance: Manufactured according to ISO, DIN, and ASTM laboratory glassware standards
Technical Specifications
- Joint Types: Standard interchangeable ground glass joints (customisation available)
- Tolerances: Precision-ground joints with standard dimensional tolerance
- Capacity Options: Additional flask volumes available on request
- Optional Accessories: Stands, clamps, vacuum tubing, cork rings, heating mantles, and filtration adapters
Handling & Precautions
- Inspect all glass components before use to ensure there are no cracks or chips.
- Assemble the apparatus on a stable support stand with all joints properly sealed.
- Use heating mantles or temperature-controlled baths; avoid direct flame heating.
- Do not expose the assembly to sudden temperature changes (thermal shock).
- Clean thoroughly using mild detergents and soft brushes—avoid abrasion.
- Allow the glassware to cool before washing or disassembling.
- Can be autoclaved within the Borosilicate Glass 3.3 temperature limits.
- Replace damaged or worn joints immediately to maintain airtight operation.
Applications
- Chemical laboratories: Routine solvent recovery and purification.
- Pharmaceutical industry: Reuse of extraction and synthesis solvents.
- Biotechnology and life sciences: Recovery of low-volume solvents in research workflows.
- Academic institutions: Teaching distillation principles and solvent handling techniques.
- Quality Control / Quality Assurance labs: Preparation and recycling of analytical-grade solvents.
- Supports cost-efficient, sustainable, and consistent lab operations by reducing solvent waste.